Introduction:
The oil and gas industry, being pivotal to global energy needs, faces increasing inquiry for its environmental impact. One such issue is the use of hazardous chemicals, such as xylene, in drilling operations.
Xylene is a widely used aromatic solvent for cleaning surface pipelines, wellbore tubing, and especially the area near the wellbore. Xylene poses serious environmental risks due to its toxic nature, contributing to air and water pollution and harming ecosystems.
The toxicity of xylene to human health and the environment has sparked concern, prompting the need for safer alternatives.Each year, billions of pounds of solvent waste are released into the environment, either through the air as vapors or in water discharge streams.
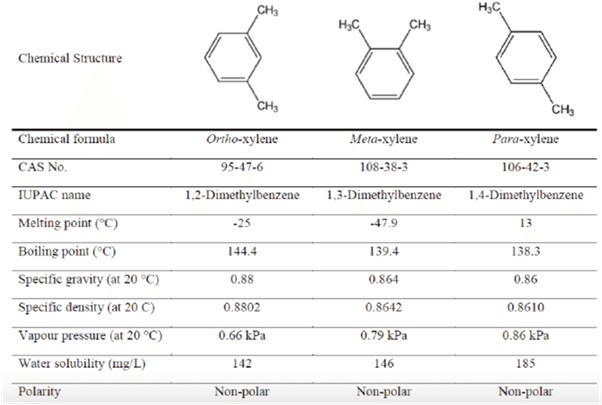
Commercial xylene is composed of three types of isomers: ortho (o-), meta (m-), and para (p-), with m-xylene making up about 45-70% of the total. The table below shows the structure and the chemical and physical properties of each isomer. The exact makeup of mixed xylenes can change, but the table illustrates the range of each type.
In response to these challenges, innovative solutions are emerging. One such solution is CleanGo, an environmentally friendly and non-toxic alternative to xylene that promises to revolutionize the way drilling operations are conducted. This research explores the viability of CleanGo as a replacement for xylene in drilling operations, examining its benefits in terms of safety, environmental impact, and cost-effectiveness.
Use of Xylene in Oil and Gas Wells:
Xylene has traditionally been used to dissolve organic deposits, but it does not alter the wettability of the rock surface, leading to only temporary results. While xylene may provide short-term effectiveness, as shown in Figure, it fails to deliver lasting solutions for long-term well maintenance.
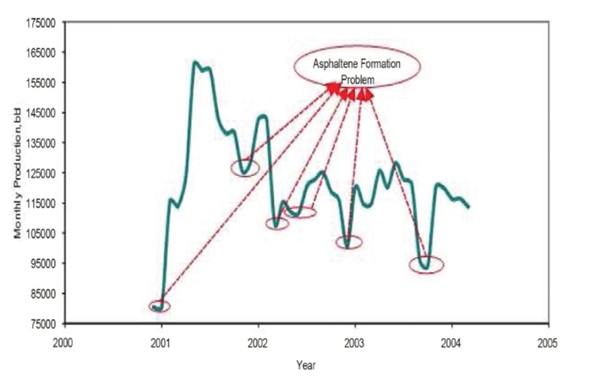
On the other hand, in one year, a Middle Eastern oil and gas producer used approximately 9,200 barrels of xylene. Figure above illustrates xylene consumption over seven years for eleven wells in a reservoir located in southern Iran (Mansoor Zoveidavianpoor, 2012).
Although acidizing is the primary method for improving production in most reservoirs, it is not effective in removing organic deposits. These deposits can block access to the target formation, reducing the effectiveness of acid stimulation. Additionally, solvents like xylene have significant limitations. They typically dissolve only about 50% of a standard downhole sample, and wellbore deposits often contain a mix of organic and inorganic materials, along with water, making complete removal more challenging (Mansoor Zoveidavianpoor, 2012).
Environmental Impact of Xylene:
Xylene can cause serious health issues. Let us discuss some of the health problems causes by xylene.
Xylene Exposure and Absorption
Xylene exposure can occur through inhalation, eye contact, ingestion, or skin absorption. On exposure the vapours are rapidly absorbed through the lungs and the slowly through the skin. Prolonged exposure to xylene leads to significant amount of solvent accumulation in the adipose and muscle tissue. About 95% of the xylene absorbed by the body is metabolized in the liver into MHA, with 70 to 80% of these metabolites being excreted through urine within 24 hours. At high concentrations, xylene acts as a narcotic, leading to neuropsychological and neurophysiological dysfunction. Respiratory issues are also common. Long-term occupational exposure to xylene has been linked to conditions such as anemia, thrombocytopenia, leukopenia, chest pain with ECG abnormalities, shortness of breath, and cyanosis, in addition to central nervous system (CNS) symptoms(Langman, 1994).
Health Effects and Metabolism of Xylene
Xylene is primarily metabolized in the liver through the oxidation of its methyl groups, followed by conjugation with glycine to form hippuric acid, which is then excreted through urine. However, high doses of xylene can damage the liver, and its metabolites can also harm hepatocytes. Some amount of xylene is eliminated through exhalation. The type and severity of health effects caused by xylene depend on various factors, such as the route of exposure, its duration, and individual differences in response to different exposure levels (Fay, 1995).
Toxicity Mechanisms and Cellular Impact
Studies indicate that acute, high-level exposure to xylene can lead to the production of CYP2E1, which contributes to the formation of oxidative intermediates and resulting cell necrosis. This mechanism is believed to be associated with short-term, high-level exposure. Another study suggests that inhaling m-xylene for six hours reduces the levels of cytochromes such as CYP2B1, 2E1, and 4B1 in the lungs, as well as 2B1 and 2E1 in the nasal mucosa.
Short-term bioassays on rats that were dermally exposed to xylene did not show mutagenic effects, but DNA fragmentation and disintegration were observed in the rats’ skin. The distribution of xylene in cells also disrupts cell membranes, leading to DNA damage and the formation of nucleases from the membrane. This disruption ultimately causes cell death due to direct and high exposure to xylene (James V. Rogers, 2001).
Environmental and Occupational Hazards
Several case studies highlight the dangers of xylene use. For example, a 2018 study conducted in Texas found that workers exposed to xylene in oil refineries had a higher incidence of respiratory issues compared to those who were not exposed. Another study in Canada revealed that xylene contamination in groundwater near oil extraction sites had detrimental effects on local wildlife, including fish and amphibians. These studies underscore the urgent need for safer alternatives to xylene in the oil and gas industry.The overall mechanism of toxicity and its harmful health effects are summarized in the figure below.
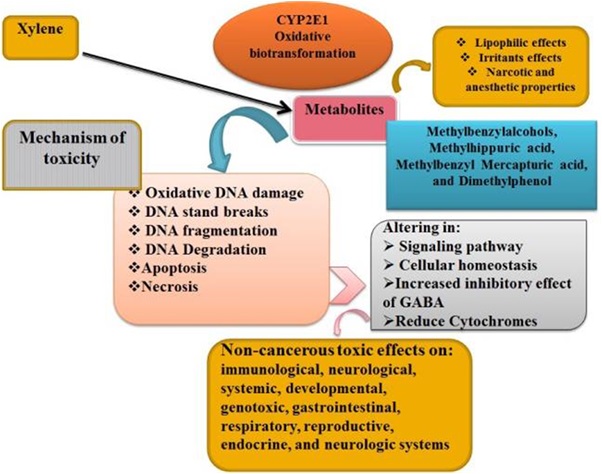
Given the toxic nature of xylene, it is clear that its widespread use in various industries poses significant risks to both human health and the environment. The exposure to high levels can cause serious cellular damage, including oxidative stress and DNA fragmentation. Moreover, chronic exposure has been linked to a range of health problems, such as respiratory issues and liver damage. These findings emphasize the need for stricter safety regulations and the exploration of safer alternatives to mitigate the harmful effects of xylene exposure.
Case Study 1: Health Impact of Xylene Exposure on Gasoline Station Workers
Study Background: A cross-sectional study was conducted to assess the exposure of gasoline station workers to xylene, with a focus on two groups: convenience store workers (CSWs), who were primarily exposed to xylene through inhalation, and filling station attendants (FSAs), who experienced both inhalation and dermal exposure. The study also included a comparison group of office workers (OWs) who were not exposed to fuel-related chemicals.
The study aimed to measure the urinary biomarkers hippuric acid (HA) and methylhippuric acid (MHA), which are byproducts of toluene and xylene metabolism, respectively. By comparing the biomarker levels in workers exposed to fuels with those in the comparison group, the study sought to assess the degree of exposure to xylene and its associated health effects.
Findings: The study revealed that gasoline station workers had significantly higher levels of HA and MHA in their urine compared to office workers, indicating higher exposure to the solvents toluene and xylene. The CSWs, who were exposed mainly through inhalation, had the highest levels of these biomarkers, followed by the FSAs, who were exposed through both inhalation and skin contact.
Workers who had higher urinary levels of HA and MHA also reported a range of health symptoms, including:
- Altered mood and depression
- Cramps
- Dizziness and drowsiness
- Headaches
- Irritability and nervousness
- Weakness
- Weight loss
These symptoms were reported more frequently by workers with elevated biomarker levels, suggesting a direct connection between xylene exposure and the onset of these health problems (Barbara R Geraldino, 2021).
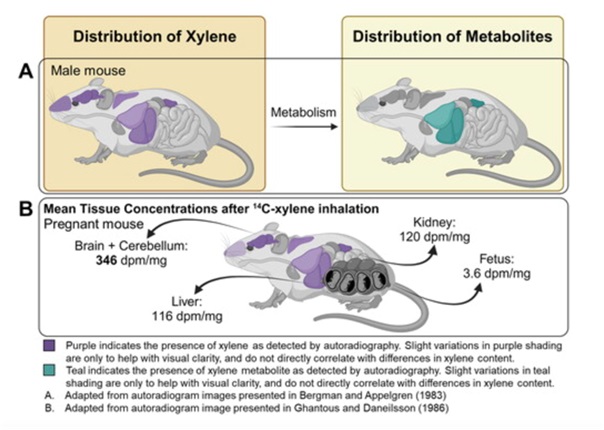
Case Study 2: Exposure of Xylene to Fetus
In this case study, pregnant mice were exposed to radiolabeled p-xylene, a volatile compound, to assess how it affects both maternal and fetal tissues. After exposure, the researchers tracked the concentration of the compound and its metabolites in both maternal and fetal tissues at multiple time points. The results showed that the concentration of p-xylene was much higher in the mother’s brain and cerebellum compared to the fetus, indicating limited fetal exposure.
At the beginning of the exposure (0 minutes), the maternal brain and cerebellum showed relatively high levels of the parent compound (p-xylene), while the fetus had minimal exposure. Over the next few hours, the concentration of metabolites in the fetal tissues increased, while the amount of unmetabolized p-xylene decreased. By contrast, in the maternal brain, the concentration of metabolites remained lower compared to the parent compound. This suggests that the maternal body was actively metabolizing p-xylene, which reduced the fetus’s exposure to the harmful compound. As the exposure time progressed, the fetus showed an increasing ratio of metabolites to unmetabolized xylene, further confirming the role of maternal metabolism in limiting fetal exposure.This study indicates that while xylene can reach the fetus, it may cause harm. The maternal body plays a protective role by metabolizing xylene, which limits the amount reaching the fetus. However, this doesn’t completely eliminate the risk, suggesting that exposure to xylene can cause serious health issues.(Frank Faulhammera, 2024).
CleanGo: Non-Toxic Alternative of Xylene
In the oil and gas industry, the use of toxic chemicals like xylene has been a standard for various cleaning and recovery operations. However, growing concerns about the environmental and health impacts of such substances have prompted the search for more sustainable alternatives. CleanGo Innovations has developed CG100, a water-based solution that addresses these concerns. CG100 is designed to reduce the toxicity and environmental impact typically associated with petroleum-based solvents, offering a safer alternative for workers and ecosystems. By enhancing oil recovery and improving acid treatment efficacy, CG100 provides a cost-effective and eco-friendly solution that doesn’t require major retrofitting of production facilities.
This formulation promotes biodegradability, ensuring minimal long-term damage to the environment. Its green certification proves adherence to environmental standards, which helps companies comply with regulations and enhance their public image. CG100 can replace traditional chemicals like xylene and Varsol in various applications, such as cleaning, degreasing, and solvent operations. The formulation’s ability to integrate smoothly with existing facilities while offering significant performance and safety advantages makes it an appealing option in an industry under increasing scrutiny for its environmental practices.CleanGo’s CG100 is not just an alternative to harmful solvents but a strategic choice for oil and gas companies seeking to meet sustainability goals while maintaining high operational efficiency.
Here’s a brief explanation of each benefit of CleanGo:
- Environmentally Friendly: CleanGo’s water-based formula ensures minimal environmental impact, unlike traditional petroleum-based solvents. It’s biodegradable, breaking down naturally without causing long-lasting harm to ecosystems, which aligns with sustainability goals in the industry.
- Safety: By significantly reducing exposure to volatile organic compounds (VOCs), CleanGo decreases the risk of health hazards such as respiratory issues or skin problems among workers, offering a safer, healthier workplace.
- Regulatory Compliance: CleanGo complies with environmental regulations, helping companies meet legal standards regarding toxic substances and pollution. This is crucial for avoiding fines and maintaining a positive reputation in the industry.
- Cost-Effective: CleanGo’s formulation is compatible with existing equipment, reducing the need for expensive facility retrofits. This saves both time and money during the transition to a more sustainable solution.
- Improved Performance: CleanGo optimizes oil recovery by reducing interfacial tension between oil and formation molecules. It works well in tandem with acid treatments, enhancing overall efficiency in production without compromising performance.
CleanGoCertifications:
- Green Seal Certified: This certification from an independent, non-profit organization ensures CleanGo’s commitment to eco-friendly practices through rigorous, science-based evaluations.
- Leaping Bunny Certified: A commitment to ethical testing, CleanGo ensures no animal testing in any phase of product development, offering reassurance to ethically-conscious consumers.
- Health Canada Registered: CleanGo has been evaluated and approved for sale in Canada, ensuring its compliance with stringent health and safety standards, backed by the DIN (Drug Identification Number).
- EPA Registered: Certified by the U.S. Environmental Protection Agency, CleanGo meets federal safety and efficacy standards for environmental protection, making it a trustworthy option for eco-conscious industries.
Discussions and Results: CleanGo vs. Xylene
Laboratory Experiments:
The laboratory experiments revealed that CleanGo performed comparably to xylene in terms of solvent efficiency and evaporation rate. However, CleanGo demonstrated significantly lower toxicity levels, making it a safer alternative for industrial use. The reduced toxicity of CleanGo was attributed to its unique chemical composition, which minimizes the release of harmful VOCs. The field trials conducted in collaboration with oil and gas companies provided further evidence of CleanGo’s effectiveness. In real-world industrial settings, CleanGo was found to be as effective as xylene in solvent extraction and cleaning applications. Additionally, the use of CleanGo resulted in a noticeable reduction in air pollution and improved workplace safety.
Stakeholder Reviews:
The stakeholder interviews revealed a strong interest in adopting CleanGo as a sustainable alternative to xylene. Industry professionals expressed optimism about the potential of CleanGo to meet regulatory requirements and improve public perception of the oil and gas industry. Environmental regulators highlighted the importance of reducing VOC emissions, while health experts emphasized the need for safer industrial chemicals. The data analysis confirmed that CleanGo is a viable alternative to xylene, with comparable performance and significantly lower toxicity. The statistical analysis revealed a strong correlation between the use of CleanGo and reduced VOC emissions, supporting its potential to mitigate the environmental and health impacts of xylene.
Conclusion
This study underscores the serious environmental and health risks posed by xylene in the oil and gas industry. Xylene, while effective in certain operations, presents significant hazards, including toxicity to human health, environmental pollution, and long-term ecological damage. Through a comprehensive review, including case studies on the adverse effects of xylene exposure on gasoline station workers and animal health, it becomes evident that alternative solutions are urgently needed.
The development of CleanGo as a non-toxic, environmentally friendly alternative to xylene marks a pivotal shift in industry practices. With its lower toxicity, compliance with environmental standards, and improved workplace safety, CleanGo offers a viable and sustainable solution. The laboratory experiments and field trials conducted show that CleanGo is equally effective as xylene in solvent extraction and cleaning operations, while significantly reducing VOC emissions and health risks. Moreover, stakeholder feedback reflects growing industry interest in transitioning to safer and more sustainable alternatives.
References:
[Online] / auth. Barbara R GeraldinoUbirani B Otero, Marcia Sarpa. – 2021. – https://pmc.ncbi.nlm.nih.gov/articles/PMC8159630/.
[Online] / auth. Fay Risher, Wilson. – 1995. – https://stacks.cdc.gov/view/cdc/6958/cdc_6958_DS1.pdf.
[Online] / auth. Frank FaulhammeraMartijnRooseboom,NeslihanAygunKocabas,Josje H. E. Arts,AlexandraCordova,ElaineFreeman,Larry G.. – 2024. – https://www.tandfonline.com/doi/full/10.1080/10408444.2024.2413073#.
[Online] / auth. James V. Rogers Palur G. Gunasekar, Carrol M. Garrett, James N. McDougal. – 2001. – https://onlinelibrary.wiley.com/doi/abs/10.1002/jbt.21.
[Online] / auth. Langman. – 1994. – https://www.tandfonline.com/doi/abs/10.1080/00313029400169711.
[Online] / auth. Mansoor ZoveidavianpoorAriffinSamsuri, Seyed Reza Shadizadeh. – 2012. – https://www.researchgate.net/publication/235006348_Health_Safety_and_Environmental_Challenges_of_Xylene_in_Upstream_Petroleum_Industry.

